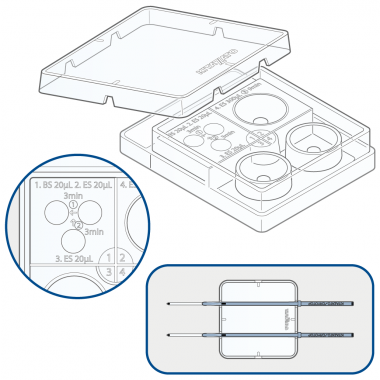
- Premarkings on the plate guide you to prepare the different solutions in appropriate volumes.
- High repeatability in the equilibration conditions leads to stable results.
- Ditches on the lid hold Cryotop stable for better handling.
Collaborative Development: Kato Ladies Clinic / Keio University, Department of. Obstetrics & Gynecology / National Center for Child Health and Development / Obstetrics and Gynecology, St Marianna University School of Medicine / Obstetrics and Gynecology, Graduate Schoold of Medicine and Faculty of Medicine, The Univertisy of Tokyo
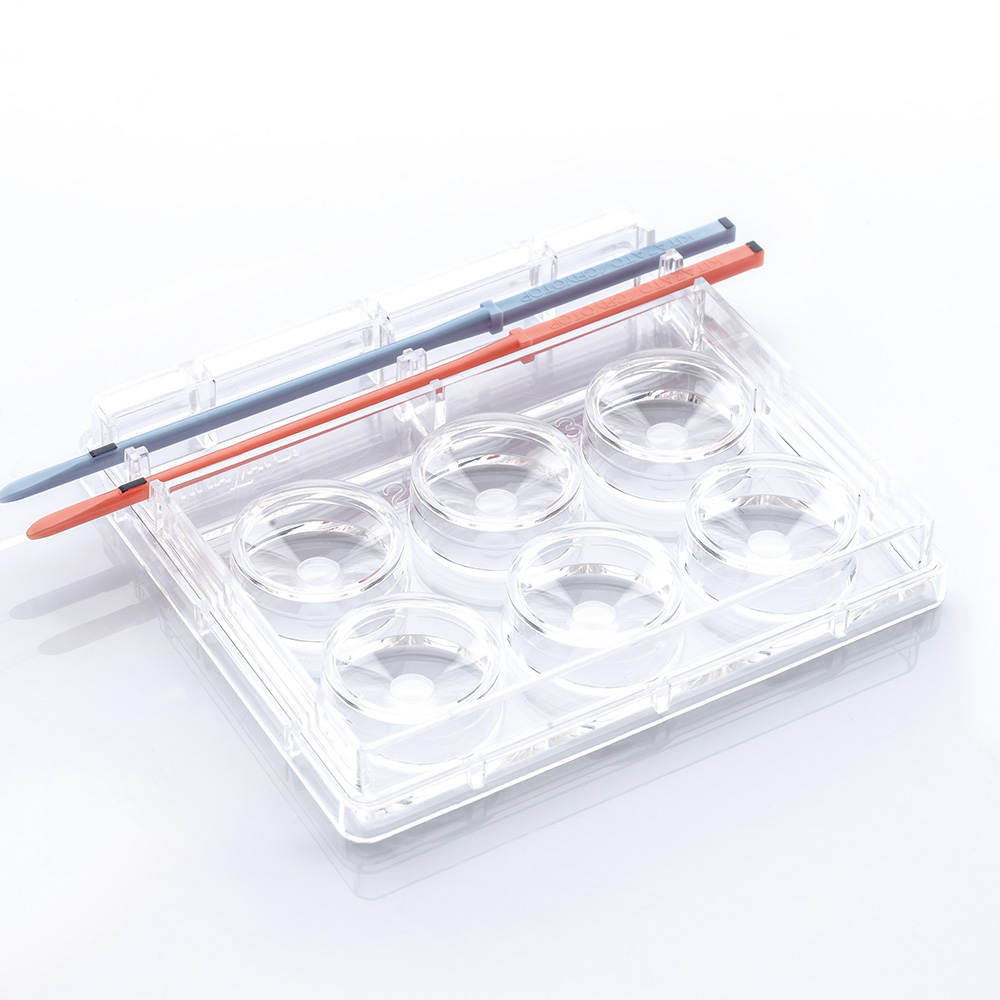
Oocyte Cryo Plate
| Product Name |
Oocyte Cryo Plate (3well) | REF | 83061 | Code | ー | Contents | 10pcs/box |
|---|---|---|---|---|---|---|---|
| Quality Control |
SAL (Sterility Assurance Level): 10-6 / Endotoxin ≤ 0.5 EU/device / MEA (Mouse Embryo Assay) | ||||||
| Storage | ー | Shelf Life | 3 years | ||||