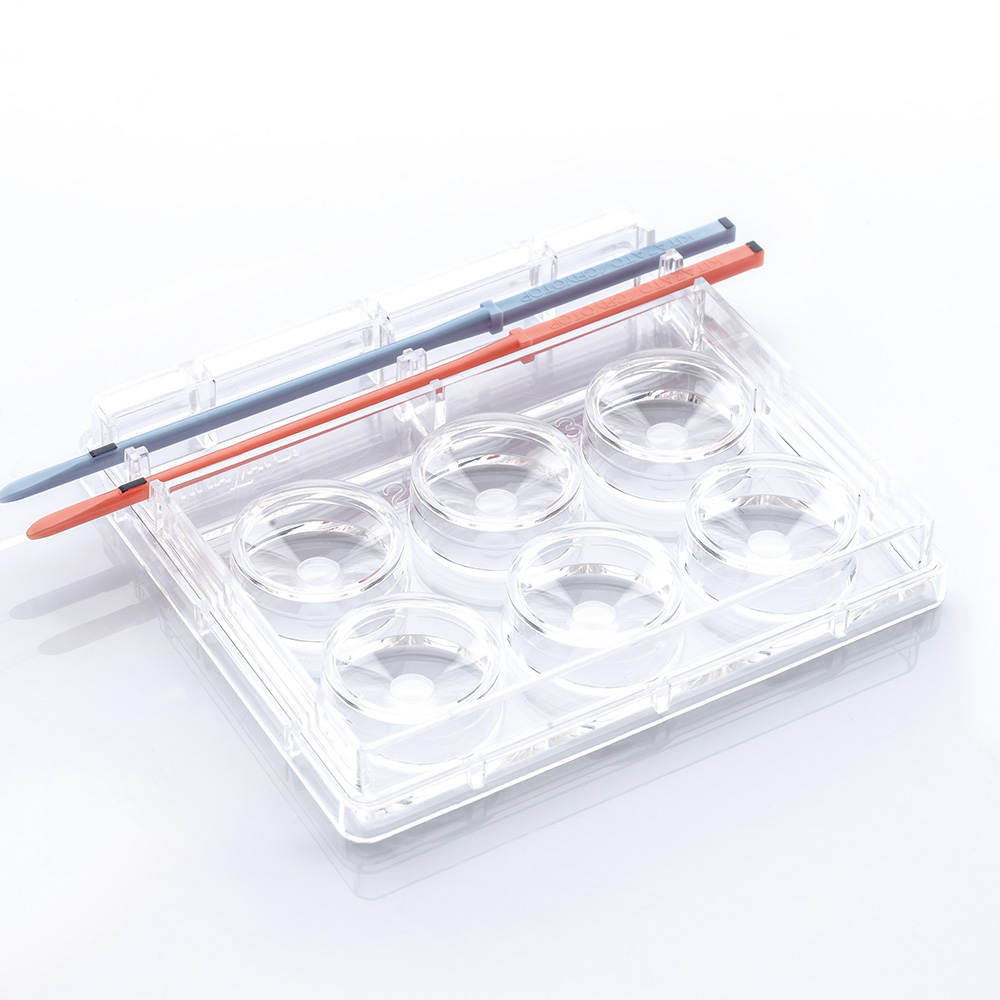
- Repro Plate is a polystyrene dish specifically designed to perform The Cryotop® Method efficiently.
Designed to accommodate the media volumes according to Kitazato vitrification and thawing protocols. - One or two rows of 3 conic-shaped wells.
- Allows to perform both vitrification and thawing procedures.
- High transparency and great visibility.
- Slots to secure Cryotop® firmly in place.
Repro Plate
| Product Name |
Repro Plate-K1 (6well) | REF | 83007 | Code | ー | Contents |
200pcs Individually Packed |
||
|---|---|---|---|---|---|---|---|---|---|
| Product Name |
Repro Plate-K1 (3well) | REF | 83010 | Code | ー | Contents | 10pcs/box | ||
| Product Name |
Repro Plate-K1 (3 well) | REF | 83011 | Code | ー | Contents |
200pcs Individually Packed |
||
| Product Name |
Repro Plate-K2 (6well) | REF | 83016 | Code | ー | Contents | 10pcs/box | ||
| Product Name |
Repro Plate-K2 (6well) | REF | 83017 | Code | ー | Contents |
200pcs Individually Packed |
||
| Product Name |
Repro Plate-K3 (3well) | REF | 83018 | Code | ー | Contents | 10pcs/box | ||
| Product Name |
Repro Plate-K3 (3well) | REF | 83019 | Code | ー | Contents |
200pcs Individually Packed |
||
| Product Name |
Repro Plate-K6 (6well) | REF | 83020 | Code | ー | Contents | 10pcs/box | ||
| Product Name |
Repro Plate-K6 (6well) | REF | 83021 | Code | ー | Contents |
150pcs Individually Packed |
||
| Quality Control |
SAL (Sterility Assurance Level): 10-6 / Endotoxin ≤ 0.5 EU/device / MEA (Mouse Embryo Assay) |
||||||||
| Storage | ー | Shelf Life | 3 years | ||||||